Mobile Menu
- Education
- Research
-
Students
- High School Outreach
- Undergraduate & Beyond: Community of Support
- Current Students
- Faculty & Staff
- Alumni
- News & Events
- Giving
- About

An international research team that includes a University of Toronto scientist is deepening our understanding of a defining characteristic in Alzheimer’s and many neurodegenerative diseases: a protein called tau.
Using advanced microscopic techniques, the researchers studied tau in rare neurodegenerative diseases and identified the protein's detailed shape in each disorder.
“This research absolutely provided a confirmation that, at the molecular level or atomic level, there are differences between the shape of the tau molecules leading to these different conditions,” says Gabor Kovacs, who contributed to the work and is a scientist in the Tanz Centre for Research in Neurodegenerative Diseases at U of T’s Temerty Faculty of Medicine.
The journal Nature published the findings in the fall of 2021. Researchers from the MRC Laboratory of Molecular Biology in the UK led the study, which involved collaborators from Japan, the U.S., the UK and the Netherlands.
“This paper opens our eyes when it comes to therapy development and points to the potential to develop very highly specific molecules, even in the direction of personalized medicine, to diagnose and treat unique disorders or unique families of disorders based on their tau shape,” says Kovacs, who is also a professor of laboratory medicine and pathobiology at U of T and co-director of the Rossy Program for Progressive Supranuclear Palsy Research at University Health Network.
In its healthy shape, tau plays a vital role. The protein supports the structure of nerve cells in the brain, acting as “the skeleton of the nerve cells,” explains Kovacs.
But, for reasons that scientists do not understand, “the three-dimensional structure of the tau protein changes and starts to build fibrils. Imagine that, instead of a healthy skeleton, one of the bones breaks and then it sticks out and forms little needle-like fibrils that accumulate,” says Kovacs.
In these harmful shapes, says Kovacs, tau is the most frequent protein that accumulates in a neuro-degenerating brain — more than 30 brain conditions show tau accumulation.
What made this study possible now is a huge technological leap — the availability of cryo-electron microscopy or cryo-EM, which enables researchers to see at a resolution that was previously impossible to imagine, says Kovacs. “It's like the infinity of the universe — researchers can now go in the other direction to see extremely small.”
Thermo Fisher Scientific, which makes cryo-transmission electron microscopes for cryo-EM research, was a study collaborator.
Using cryo-EM, the study team examined post-mortem brain tissue from 18 people with eight rare neurodegenerative diseases and their clinical subtypes, including progressive supranuclear palsy, which is one of the most common tau-related diseases after Alzheimer’s disease but affects a small fraction of people by comparison.
The study found that tau fibrils are indeed different among disorders. “These differences might be the reason why, for example, in an Alzheimer's disease brain, the tau protein propagates in and affects different brain regions compared to a disease with movement disorder, because different types of nerve cells in the brain are more protective against or susceptible to different tau shapes.”
The findings validate what doctors and brain scientists understood about tau-related disorders based on patients' symptoms and earlier research into harmful tau buildups: differences characterize specific disorders. The newly identified variations in tau shapes also confirm these differences.
The team also showed that while some disorders have a unique tau shape, others have a similar shape. For example, progressive supranuclear palsy and globular glial tauopathy have a similar tau shape, which differs from that in Alzheimer’s disease.
Based on their findings, the researchers have proposed a new system for classifying tau-related neurodegenerative diseases, defining those with a unique shape as individual diseases and those with a common shape as a family of diseases. Kovacs says the new findings and disease classification will drive research to develop more precise diagnosis methods and treatments for individual diseases or families of diseases.
There are no cures for tau-related disorders to date. While doctors can test for tau in a patient’s cerebrospinal fluid to diagnose Alzheimer’s disease, current tests cannot specify which disorder a patient has for other tau-related disorders. "The future is to go into much more detail and precision,” Kovacs says.
Harmful Tau Buildups
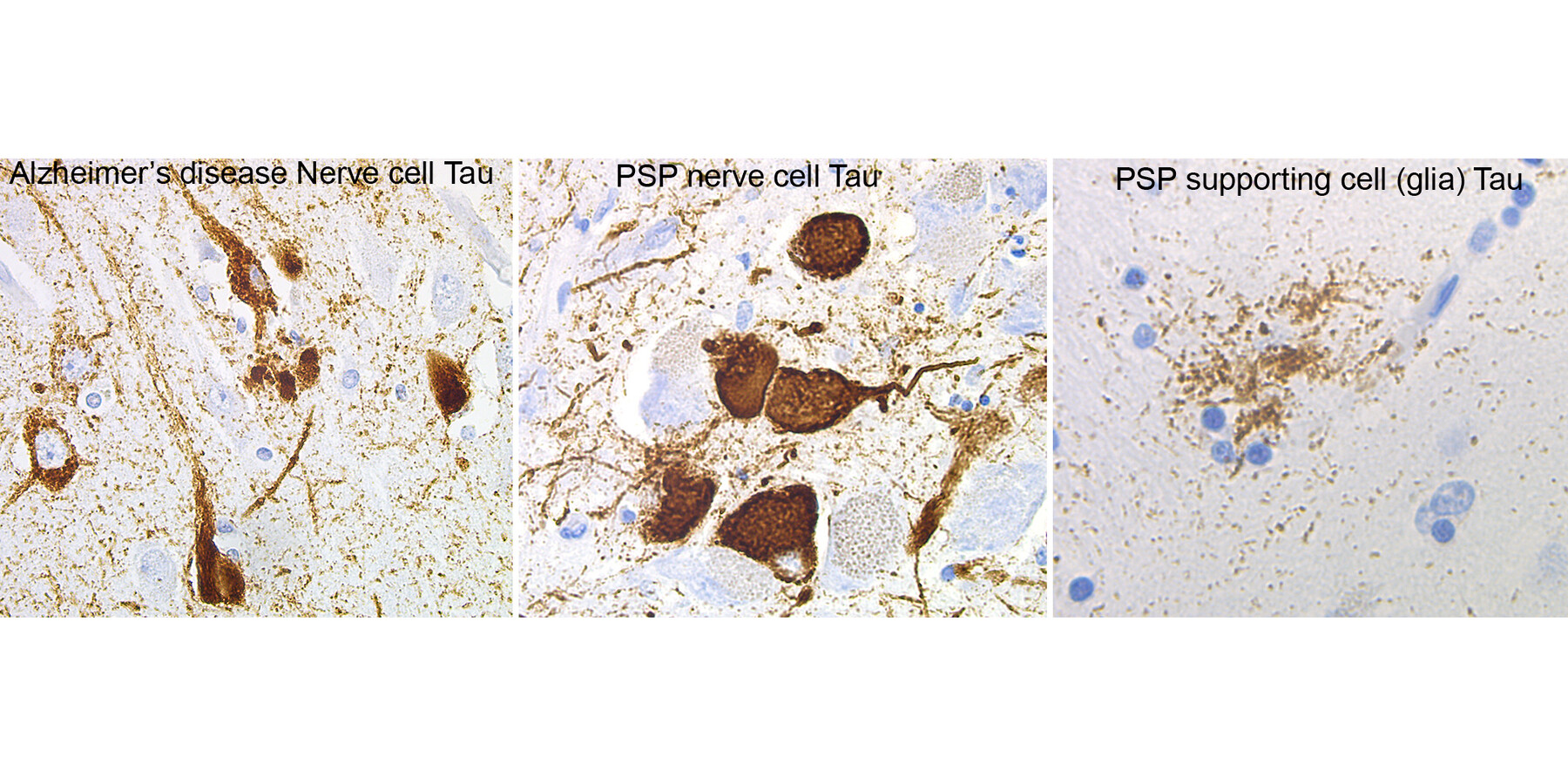
(From left) The brown markings show harmful buildups of the tau protein in nerve cells from a person with Alzheimer’s disease, nerve cells from a person with progressive supranuclear palsy (PSP) and in a supporting glia cell from a person with PSP.
In a recent study, the researchers — including Gabor G. Kovacs in the Tanz Centre for Research in Neurodegenerative Diseases — went a step further, using even more advanced microscope technology to identify the different shapes of tau (not shown above).