Main Second Level Navigation
Dec 6, 2022
Researchers Uncover Molecular Vulnerability in Childhood Brain Cancer and Identify Treatment for It
Research
An unbiased metabolomics study offers hope for treating brain tumours in children while minimizing harm to cognitive development and function
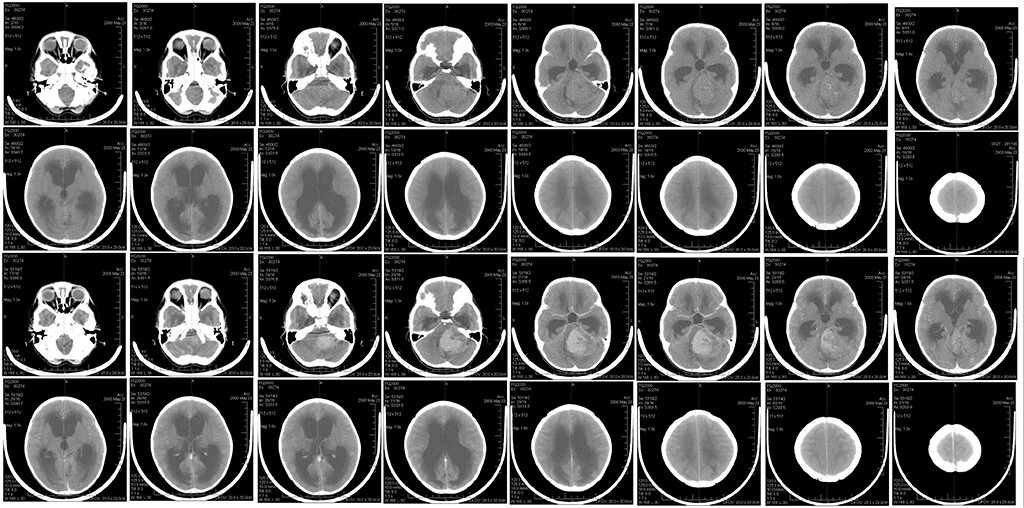
Wikimedia Commons
A brain scan of child with medulloblastoma tumour



